Still going strong
A choice of one tablet, once monthly1 or a quarterly IV injection2
Because compliance
is important
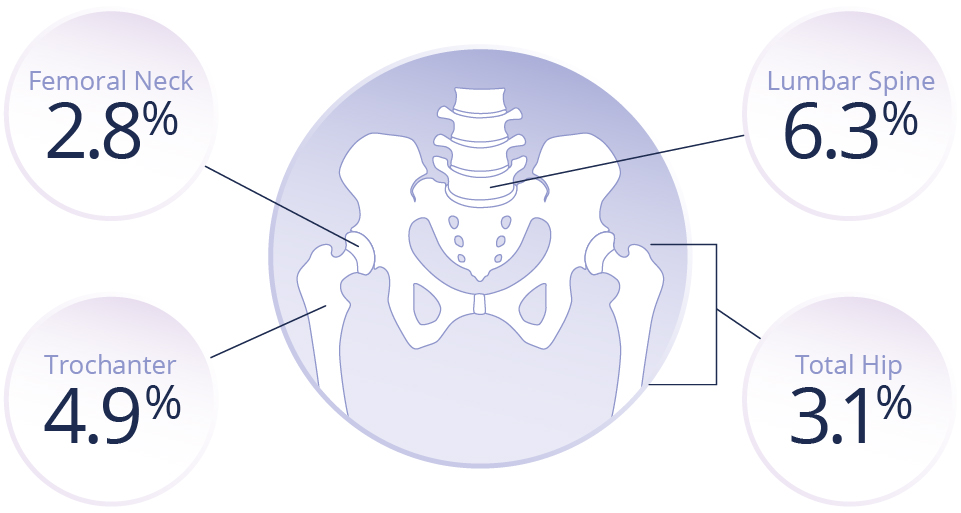
For treatment of osteoporosis in post-menopausal women at increased risk of fracture1,2